https://als.lbl.gov/toward-control-spin-states-molecular-electronics/
SCIENTIFIC ACHIEVEMENT
Researchers demonstrated, via x-ray absorption spectroscopy, that a molecule’s spin state can be reversibly switched at constant room temperature by magnetism.
SIGNIFICANCE AND IMPACT
The results represent a major step toward the goal of programmable, nanoscale molecular electronics for high-speed, low-power, logic and memory applications.
Downsizing: How low can we go?
To squeeze more information into smaller spaces, we will need to downsize from microchips to the nanoscale: the molecular level. Compared to bulk silicon, organic molecules have many advantages when used as building blocks for memory and logic components. They can be implemented as flexible thin films, they can be easily printed, and their potential switching speed is high, while their power requirements are low. But the field of molecular spintronics is still very young, and before its promise can be realized, scientists need a fuller understanding of the fundamental physics in play.
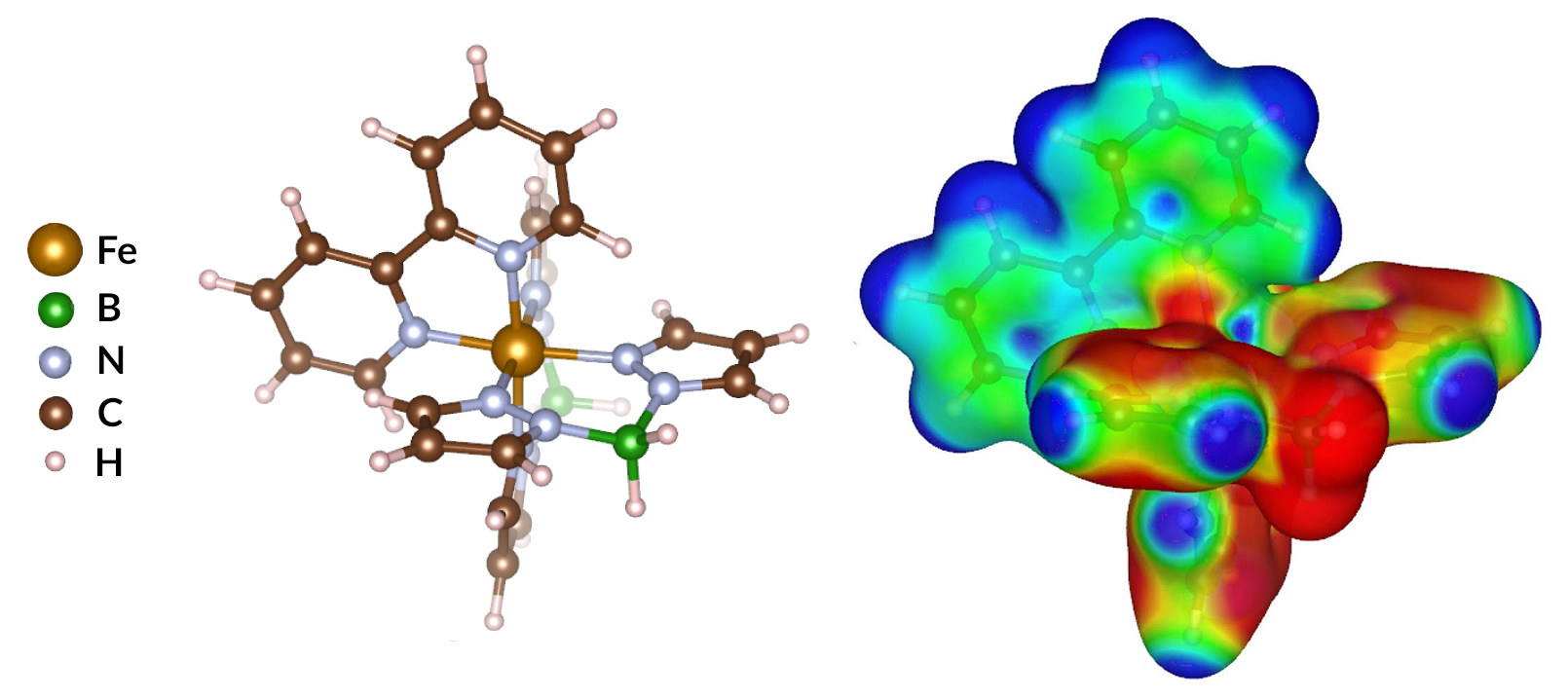
A molecule with crossover appeal
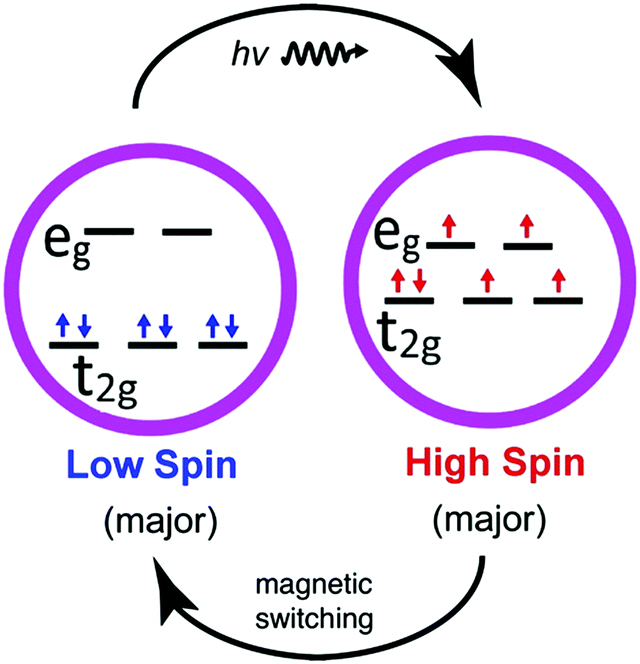
In the molecule studied here—[Fe{H2B(pz)2}2(bipy)]—the spin state is determined by the configuration of the central metal’s outer electrons (i.e., the Fe d-orbital electrons). The presence of the surrounding organic ligands splits the Fe d orbitals. If the splitting is large, the electrons will pair up in the lower orbitals (a low-spin state). If the splitting is small, the electrons can spread out over both levels (a high-spin state). For some classes of molecules, transitions from low- to high-spin states (and vice versa) can be triggered. This “spin crossover” phenomenon is a promising functionality that may be suitable for application in molecular spintronic devices.
For memory applications, there is a strong need to identify mechanisms to lock and unlock the spin state. Previous work had shown that the spin state of [Fe(H2B(pz)2)2(bipy)] can be locked in a largely low-spin configuration up to temperatures well above its thermal spin crossover temperature (160 K), by appropriate design of the molecule’s electrostatic and chemical environment (e.g., growing thin films of the molecule on a nonconducting oxide substrate). It was also found that exposure to x-rays excites the “locked” low-spin system to a high-spin state, and heating slightly above room temperature restores the low-spin state. Ultimately, the goal is direct control of the spin state via an external voltage at constant room temperature.
A missing link: magnetoelectric coupling
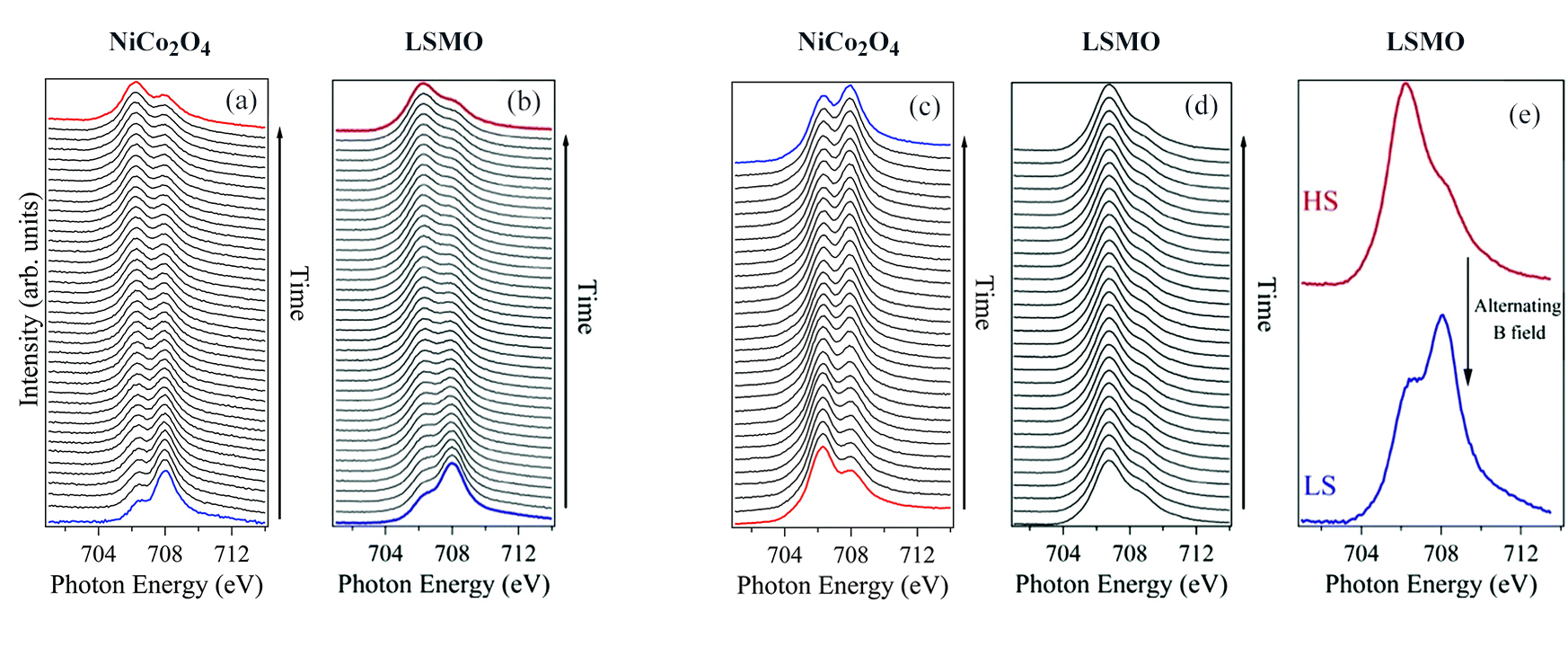
In this work, the researchers explored how the spin state of thin-film [Fe(H2B(pz)2)2(bipy)], grown on various substrates, is affected by an oscillating magnetic field. At ALS Beamline 6.3.1, x-ray absorption spectroscopy (XAS) measurements were performed at the Fe L3 and L2 edges.
The results showed that, on a substrate of NiCo2O4, alternating the direction of the magnetic field assists in the relaxation of the excited high-spin state, even with continued x-ray irradiation of the sample. However, on a substrate of La0.67Sr0.33MnO3 (LSMO), relaxation under the oscillating magnetic field only occurred in the absence of the x-ray beam—evidence that magnetic coupling with the substrate is involved. More specifically, coupling of the substrate’s local magnetic moment with the ligand electrostatic field is somehow involved in reversing the molecular spin state.
This work affirms that metal–organic molecular complexes allow the possibility of nanometer-scale, low-power, high-speed, magnetoelectric logic or memory devices. In addition, the work reveals very interesting physics that calls for further investigation, both experimentally and with advances in theory.
Researchers: X. Zhang, X. Jiang, X. Zhang, Y. Yin, X. Chen, X. Hong, X. Xu, and P.A. Dowben (University of Nebraska) and A.T. N’Diaye (ALS).
Funding: National Science Foundation. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES).
Publication: X. Zhang, A.T. N’Diaye, X. Jiang, X. Zhang, Y. Yin, X. Chen, X. Hong, X. Xu, and P.A. Dowben, “Indications of magnetic coupling effects in spin cross-over molecular thin films,” Chem. Commun. 54, 944 (2018), doi:10.1039/C7CC08246K.

No comments:
Post a Comment